COVID-19 Vaccination FAQs
Scroll down to read FAQs.
Get access to your electronic vaccination card
Hear more stories from SEIU members who’ve gotten vaccinated.
Find out where you can get vaccinated. It’s free.
Booster Shot Needed Against Omicron

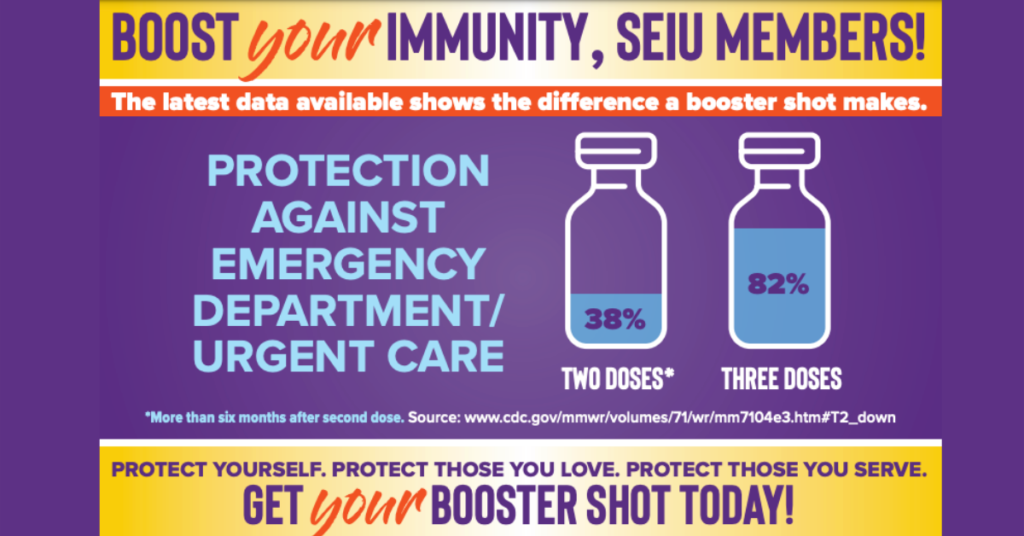
COVID Booster Shot is Like a Seatbelt

Vaccines are highly effective against COVID and COVID variants. In breakthrough cases, the vaccine still provides strong protection against serious illness and death.
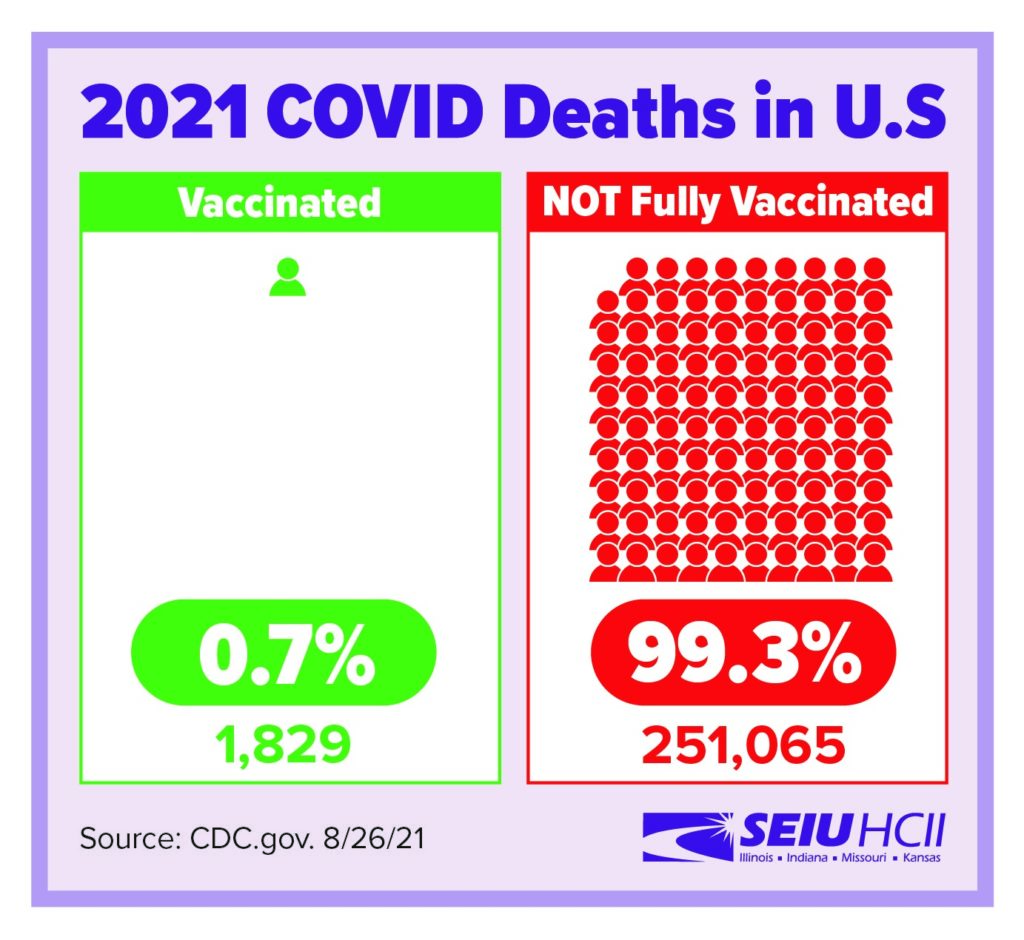
https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html

The Delta Variant Is Much More Dangerous
Source: U.S. Centers For Disease Control (CDC), updated: August 6, 2021
On July 27, 2021, CDC released updated guidance on the need for urgently increasing COVID-19 vaccination coverage and a recommendation for everyone in areas of substantial or high transmission to wear a mask in public indoor places, even if they are fully vaccinated. CDC issued this new guidance due to several concerning developments and newly emerging data signals. First is a reversal in the downward trajectory of cases. In the days leading up to our guidance update, CDC saw a rapid and alarming rise in the COVID case and hospitalization rates around the country.
Infections and Spread
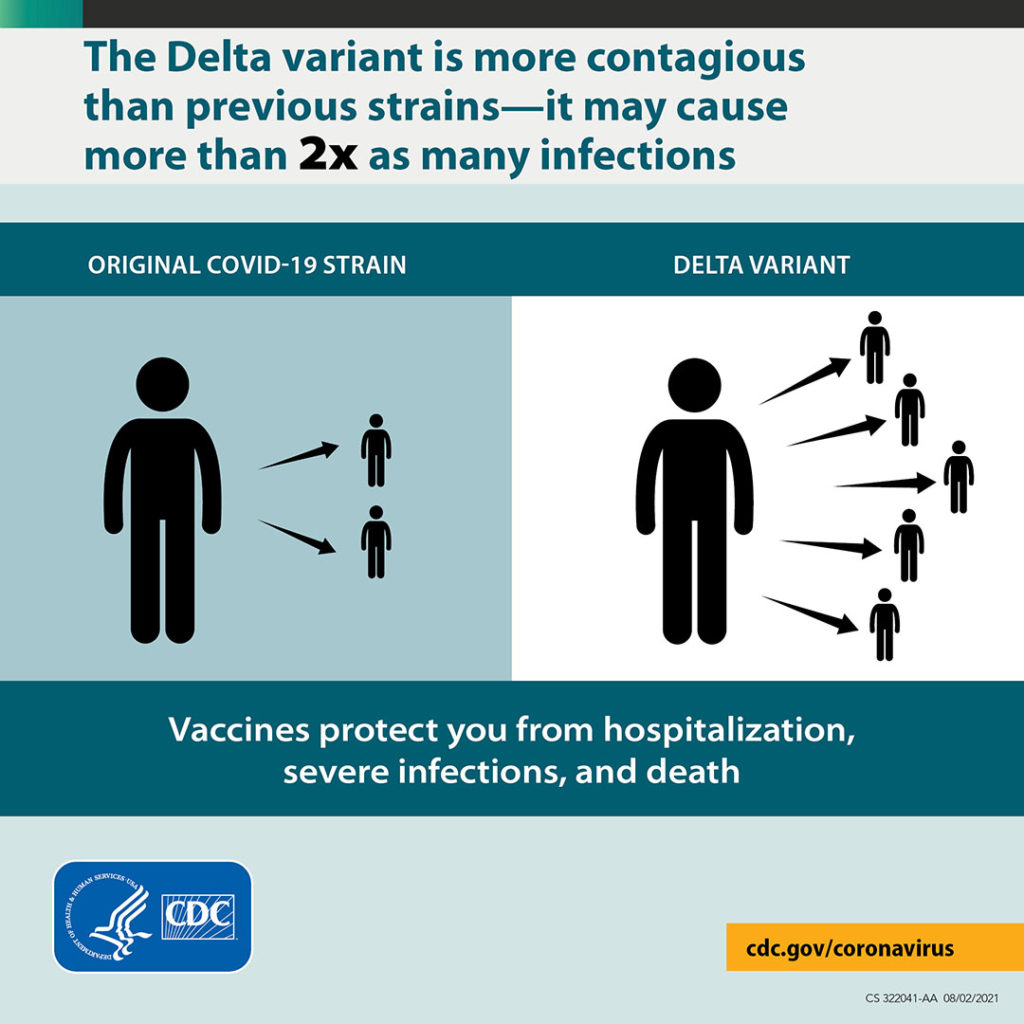
- The Delta variant is more contagious: The Delta variant is highly contagious, more than 2x as contagious as previous variants.
- Some data suggest the Delta variant might cause more severe illness than previous strains in unvaccinated persons. In two different studies from Canada and Scotland, patients infected with the Delta variant were more likely to be hospitalized than patients infected with Alpha or the original virus strains.
- Unvaccinated people remain the greatest concern: Although breakthrough infections happen much less often than infections in unvaccinated people, individuals infected with the Delta variant, including fully vaccinated people with symptomatic breakthrough infections, can transmit it to others. CDC is continuing to assess data on whether fully vaccinated people with asymptomatic breakthrough infections can transmit. However, the greatest risk of transmission is among unvaccinated people who are much more likely to contract, and therefore transmit the virus.
- Fully vaccinated people with Delta variant breakthrough infections can spread the virus to others. However, vaccinated people appear to be infectious for a shorter period: Previous variants typically produced less virus in the body of infected fully vaccinated people (breakthrough infections) than in unvaccinated people. In contrast, the Delta variant seems to produce the same high amount of virus in both unvaccinated and fully vaccinated people. However, like other variants, the amount of virus produced by Delta breakthrough infections in fully vaccinated people also goes down faster than infections in unvaccinated people. This means fully vaccinated people are likely infectious for less time than unvaccinated people.
Vaccines effective against Delta
Vaccines in the US are highly effective, including against the Delta variant
- The COVID-19 vaccines authorized in the United States are highly effective at preventing severe disease and death, including against the Delta variant. But they are not 100% effective and some fully vaccinated people will become infected (called a breakthrough infection) and experience illness. For such people, the vaccine still provides them strong protection against serious illness and death.
Learn more about mask guidance from the CDC.
What About Vaccine Side Effects?
“[The vaccine] does its job and it’s out of there,” says Dr. Heather Prendergast. That’s why she’s confident it’s safe in the long-term for patients. Share this video with others.

Pfizer Vaccine Fully FDA Approved
On Audust 23, 2021, the Pfizer-BioNTech’s COVID Vaccine recieved full approval from the FDA.
Many of us have been waiting to see how safe and effective these vaccines were. With the more dangerous Delta variant raging and with FDA approval of the Pfizer vaccine, there’s no more time and no more reason to wait. Controlled clinical trials conducted on people around the world show the Pfizer vaccine is 91% effective in preventing COVID and real world experience from tens of millions shows it’s even more effective in preventing severe cases and death.

Vaccines have been vigorously tested. mRNA technology in COVID vaccines has been around for decades
mNRA technology used in COVID vaccines has been around since the 1990s. We’re lucky this technology was there when we needed it most.
The vaccines have gone through the proper steps in the regulatory process. Clinical trials are evaluating investigational COVID-19 vaccines in tens of thousands of study participants to generate the scientific data and other information needed by FDA to determine safety and effectiveness.
As of August 17, 2021, more than 4.7 billion COVID vaccines (358 million in the U.S.) have been administered worldwide.
Learn more about the emergency authorization process.
What’s in the vaccine? How does it work?
Pre-Existing Conditions

Johnson & Johnson Update
updated 4/26/21
Is the Johnson & Johnson vaccine safe? Yes.
The CDC & FDA recommend its use
The U.S. Centers for Disease Control (CDC) and Federal Drug Administration (FDA) have recommended that use of Johnson & Johnson’s Janssen (J&J/Janssen) COVID-19 Vaccine resume in the United States, effective April 23, 2021.

Rare Risk of Blood Clots
However, women younger than 50 years old should be aware of the rare risk of blood clots with low platelets after vaccination, and that other COVID-19 vaccines are available where this risk has not been seen.
- There is a plausible causal relationship between J&J/Janssen COVID-19 Vaccine and a rare and serious adverse event—blood clots with low platelets (thrombosis with thrombocytopenia syndrome, or TTS). However, after reviewing all available safety data, CDC and FDA recommend use of this vaccine resume in the United States given that the known and potential benefits outweigh the known and potential risks.
- This adverse event is rare, occurring at a rate of about 7 per 1 million vaccinated women between 18 and 49 years old. For women 50 years and older and men of all ages, this adverse event is even more rare.
If you get the Johnson & Johnson Vaccine here’s what you should look out for

- For three weeks after receiving the vaccine, you should be on the lookout for possible symptoms of a blood clot with low platelets. These include:
- Severe or persistent headaches or blurred vision
- Shortness of breath
- Chest pain
- Leg swelling
- Persistent abdominal pain
- Easy bruising or tiny blood spots under the skin beyond the injection site
Seek medical care right away if you develop one or more of these symptoms.
Learn more
Vaccine Overview
SEIU members are essential workers, on the front lines of the fight against the pandemic that has ravaged our communities. As we confront the triple crises of the pandemic, the economic collapse, and ongoing racial injustice, we demand that corporations and elected leaders not just praise us, but respect us, protect us, and pay us. Because we fight for safety, justice, and equity for ourselves, our families, the people we care for and provide service to, and for our communities as a whole, we commit to the following principles regarding the COVID19 vaccine:
- Vaccines are a proven technology to prevent the spread of disease. The COVID vaccine is a critical tool to protect our families, ourselves, and our communities as we fight to put an end to this deadly virus. We encourage SEIU members to take the vaccine.
- Vaccine distribution must be equitable and transparent and must prioritize communities hardest hit by the virus, including essential workers, people with underlying health conditions and disproportionately impacted communities of color.
- Vaccines must be provided free of charge, and workers should be provided with paid time off if the vaccination process requires them to miss work.
- Employers must not use vaccines as a substitute for worker safety and infection control protocols nor for ensuring access to personal protective equipment.
- Vaccine distribution plans must include education and outreach activities that involve essential workers and our communities deeply and meaningfully.
- Outreach and distribution plans must recognize the impact of structural racism in causing trauma and heightened levels of distrust about vaccination in Black and brown communities.
- The best approach to encouraging universal vaccination is through education and outreach, not through making vaccination mandatory.
What’s in the vaccine? How does it work?
Moderna & Pfzier Vaccines
There are two vaccines that are or could soon be available, and both use messenger RNA (mRNA) technology. Unlike other vaccines, mRNA technology does not use any live virus particles. You will not be exposed to the virus that causes COVID-19.
Instead, the vaccines contain instructions for your cells. The messenger RNA — a piece of genetic code — tells your cells to make the COVID-19 spike protein themselves. Once your cells make the spike protein, your immune system will create the antibodies that fight COVID-19 and protect you from getting sick from this virus, providing a significant level of immunity.
To be effective, both of the vaccines require you to receive two shots, given a few weeks apart.
Johnson & Johnson
The ultimate difference is the way the instructions are delivered. The Moderna and Pfizer vaccines use mRNA technology, and the Johnson & Johnson vaccine uses the more traditional virus-based technology.
mRNA is essentially a little piece of code that the vaccine delivers to your cells. The code serves as an instruction manual for your immune system, teaching it to recognize the virus that causes COVID-19 and attack it, should it encounter the real thing.
Instead of using mRNA, the Johnson & Johnson vaccine uses a disabled adenovirus to deliver the instructions. This adenovirus is in no way related to the coronavirus. It is a completely different virus. Although it can deliver the instructions on how to defeat the coronavirus, it can’t replicate in your body and will not give you a viral infection.
Can I get COVID-19 from the vaccine?
No. There are no live virus particles. While you might feel minor, temporary side effects from the injection, it is impossible to contract the virus from the vaccine.
Will the vaccine cause side effects? If so, how long might they last?
Some people who get a COVID-19 vaccine will experience side effects, particularly after a second dose. The side effects of the vaccine appear to be minor and temporary. Participants have reported pain at the injection site, fatigue, and occasional fever, headache, or aching muscles and joints. These side effects fade within 1-2 days.
These side effects are actually common with all vaccines: they are a sign that a vaccine is working and triggering an immune response.
If someone is going to have a bad reaction to a vaccine, it is likely to occur in the first six weeks after vaccination.
Are there any long-term side effects?
COVID-19 vaccines are still being tested for long-term side effects. At this point, no long-term safety issues have been detected. The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) are monitoring closely. As more people get vaccinated, more information will be available in the coming weeks and months. CDC scientists and medical professionals will be continuously reviewing vaccine safety. They will keep providing information to the public and will take action on new safety concerns if needed.
But even though we are still learning about COVID-19 vaccines, here’s what we do know for sure: getting sick with COVID-19 is dangerous. We know that COVID-19 can cause long-term health problems, even in mild cases. It is unlikely that we will find any vaccine-related side effects that are riskier than actually having COVID-19.
Has anyone died or become ill after taking the vaccine?
No. There are two vaccines – one is from Pfizer and the other is from Moderna. Nearly 73,000 individuals took part in clinical trials for the two vaccines. There were no deaths, and nobody reported severe illness following the vaccination.
How effective is the vaccine?
Both vaccines have a very high level of effectiveness: Pfizer has a 95 percent rate and Moderna has a 94 percent rate. That means that among people who took the vaccines, there were 94 to 95 percent fewer cases of COVID-19 than among those who did not receive it.
While it’s difficult to compare vaccines for different diseases, for context, flu vaccines are only 40-60% effective in any given year. The high level of effectiveness of the COVID-19 vaccine means it has the potential to significantly prevent the spread of the disease.
Is one vaccine better than the other?
No. The two vaccines use the same mRNA technology, and they have similar levels of effectiveness: among people who took the vaccines, there were 94 to 95 percent fewer cases of COVID-19 than among those who did not receive it. To be effective, both of the vaccines require you to receive two shots, given a few weeks apart.
Your employer may administer one of the two authorized vaccines, depending on their supply. Once you receive the first dose, you cannot get a second shot from a different vaccine.
How many doses do I need to be fully protected? Is one good enough?
To be effective, both vaccines require two shots, given a few weeks apart. It is typical for the second dose of vaccine to give a more significant, longer-term boost. Giving a vaccine in two doses is common for many childhood vaccines. The first shot primes the immune system, helping it recognize the virus, and the second shot strengthens the immune response. Pfizer’s second shot is given 21 days after the first one; Moderna’s is 28 days later.
Can I mix and match vaccines?
No. For a two-dose vaccine, your second dose must be from the same vaccine as the first. Since the vaccines differ in composition, storage and time between the two doses, experts say people must take the same vaccine for both doses.
Can I still get the virus even if I take the vaccine?
Yes. It typically takes a few weeks for the body to build immunity after vaccination. That means it’s possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and get sick. This is because the vaccine has not had enough time to provide protection.
While the vaccine provides significant protection, it is not 100% effective. There is a slight chance you may still get infected, but it will most likely be a mild case of the virus as opposed to a severe case which is possible without the vaccine. Contracting the virus without protection can have potentially deadly consequences – taking the vaccine does not.
Can I still spread the virus even after getting vaccinated?
We don’t yet know whether vaccinated individuals can spread the virus to others who may not have received the vaccine. That’s why it will be critical that everyone continues to wear masks, socially distance and follow all the necessary public health protocols both at work and elsewhere.
Does the vaccine work better depending on age, weight, or race?
Based on the available data, we know the Pfizer vaccine works well regardless of age, weight or race. Data on the Moderna vaccine is expected to be released soon and we anticipate it will show similar results. Trials for both vaccines included over 25,000 people from the communities most impacted by COVID-19, including Black, Latinx, and older people.
I have pre-existing conditions. Will taking the vaccine have harmful effects?
We don’t yet know for certain how individuals with different pre-existing conditions will react to the vaccine. It is clear, however, that those with other health complications are at a higher risk for contracting severe cases of the disease. If you have a pre-existing condition, you should consult your doctor on what’s best for you.
I already had COVID-19 – do I still need a vaccine?
There is not enough information currently available to say if or for how long after infection someone is protected from getting COVID-19 again. Early evidence suggests that natural immunity from COVID-19 may not last very long, but more studies are needed to better understand this. The CDC has not issued a recommendation on whether people who had COVID-19 should get a COVID-19 vaccine.
Will I still need to wear PPE and follow public health protocols even after getting the vaccine?
Yes. We will still need to wear masks and practice physical distancing until a large proportion of the population is vaccinated and we are sure the vaccine provides long-term protection. Initially, we will not have enough vials to vaccinate everyone who wants the vaccine and the virus will still be transmitted.
While the vaccine provides significant protection, it is not 100% effective. We also don’t know whether vaccinated individuals can still carry and spread the virus to people who haven’t been vaccinated. Everyone should continue to wear PPE and follow public health protocols both at work and elsewhere.
The vaccines were made so quickly – how do I know if it’s safe and not rushed?
The mRNA vaccines produced by Pfizer and Moderna are faster to develop because they are not using live virus particles. Instead, the mRNA is easy to make in the laboratory – saving several years for development.
These vaccines are carefully studied, tested, and regulated before they can be used. The companies that created the vaccines submit extensive applications to multiple government agencies and independent bodies of scientific experts, which will only permit the vaccine to be used if the evidence shows it is safe.
Healthcare workers will be among the first who can take the vaccines. How robust were the trials? How many people were involved and how thorough was the study?
In clinical trials for the vaccine candidates from Pfizer and Moderna, over 73,000 people from the U.S. and around the world received injections of the vaccine. Both vaccines have a very high level of effectiveness.
Did the clinical trials for the vaccines from Pfizer and Moderna include people from the groups most affected by COVID-19, especially Black, Latinx, and older people?
Yes. While vaccines work the same in people of different races or ethnicities, it is important to make sure vaccines are tested in diverse population groups before they are released. The clinical trials conducted by Pfizer and Moderna included over 25,000 people from the communities most impacted by COVID-19, including Black, Latinx, and older people.
Did President Trump pressure vaccine companies or the FDA to speed up the process?
No. Public health leaders including Dr. Anthony Fauci are carefully monitoring the vaccine process, and it has moved forward without interference by President Trump and Republicans. The companies that created the vaccines submit extensive applications to multiple government agencies and independent bodies of scientific experts, which will only permit the vaccine to be used if the data and the evidence show it is safe for people. There is no time limit on the process, and nobody — not even the President — can rush it.
How does the vaccine approval process work?
In the United States, vaccines must be approved by the Food and Drug Administration (FDA) before they can be used. The FDA bases its decision to approve or not approve a vaccine on data from clinical trials. The data is reviewed by independent experts who are not part of the government or the pharmaceutical companies, and by career scientists and physicians at the FDA who are not politically appointed and who are experts in vaccine safety and effectiveness.
The scientists look out for unexpected side effects that the vaccine might have caused. This helps determine the vaccine’s “safety.” In general, the fewer and less severe the side effects are, the more the vaccine is considered safe. If the clinical trial data shows enough evidence of efficacy and safety, the FDA will approve the vaccine and license it for use in the United States.
I hear that the FDA is granting EUA status to the Pfizer COVID-19 vaccine. What does that mean?
Sometimes, the FDA will allow a medical product that has not yet been fully approved to be used in an emergency to diagnose, treat, or prevent a serious illness. This is called “emergency use authorization” or “EUA.” An Emergency Use Authorization (EUA) may be issued when the FDA determines that the product “may be effective” against the disease based on all the available scientific evidence. This is a lower standard than what’s required for full approval of a product, but it still uses early data gathered from clinical trials.
Can the government or my employer force me or other healthcare workers to take the vaccine? What about my patients – will they be forced to take it?
No, it is not mandatory for healthcare workers or patients to take the vaccine. However, healthcare workers are encouraged to take it given their frequent contact with COVID-19 patients, as well as to protect loved ones and neighbors. While healthcare workers will be given the first opportunity to take it due to their work, the general population will be eligible to take it soon after. Mass vaccination is the best way to stop the spread of COVID-19, save lives and begin to resume normalcy once again.
Will those who are vaccinated be assigned to work with patients with COVID-19 more frequently?
No, the immunization status of a healthcare worker will not affect his/her work assignment.
Is the vaccine free? Will my insurance cover it?
You will not have to pay for the vaccine. The vaccine itself is free, and Medicare, Medicaid, and Private Insurance have agreed to offer it for free. Individuals who are uninsured may incur a cost for vaccine administration.
Will I have a chance to take the vaccine later if I decline the first opportunity?
We don’t have all the details yet on how many doses will be available in the initial distribution. Due to limited doses of the vaccine, choosing not to take it when it is first available may mean you will have to wait many more months to have an opportunity to do so again.
